Automating Product Return Processing for a Leading Pharmaceutical Manufacturer
Business Problem/ Scope of Work
Goal: The goal of the CDAS/CDP project is to streamline the clinical data collection process, curate it for stakeholders, and generate smarter insights through machine learning analysis to improve clinical outcomes.
As sponsors and contract research organizations (CROs) strive to advance medical research, they are faced with the challenge of proactively assessing and mitigating risks. One area where this is particularly difficult is in the field of clinical research. Clinical research associates (CRAs) are tasked with a logistically challenging job that includes large amounts of searching, organizing, planning, and follow-up. Unfortunately, there is no clear leader in the clinical data repository space that can make this process easier for them.
To address this problem, BDF Product Reference (Mainframe Re-Platform [MFRP]) was developed. The goal of this project was to migrate legacy programs running on Mainframe to process wholesale transactions and surrounding referential data (outlet, product, and distributor) into a modern technology platform. By simplifying the process of clinical research logistics, this project aims to reduce the burden on CRAs and make it easier for sponsors and CROs to proactively assess and mitigate risks in the field of clinical research.
Business Solution
Our company has developed a new software-as-a-service (SaaS) platform for clinical data analytics. This platform allows users to combine structured and unstructured data into one standardized ecosystem for analysis. The platform is called Clinical Data Analytics Suite (CDAS) and it offers modules that provide flexibility to meet a range of customer needs.
One of the key features of CDAS is the ability to support clinical operations and data management within a single solution. This makes it a valuable tool for healthcare organizations looking to streamline their data management and analysis processes.
In addition to CDAS, our company is also working on a project called Mainframe Re-Platform (MFRP). This project aims to migrate core business processes that are currently implemented on IBM mainframes to a newer, open-source technology-based BigData platform. This will allow for greater scalability and cost-efficiency.
To ensure that MFRP is integrated into the timeline, we will be working closely with the teams at Big Data Factory (BDF) to identify and implement the appropriate components. This will ensure that the project is completed in a timely and efficient manner.
Technical Solution
The CDAS/CDP project is designed to provide a comprehensive solution for managing clinical data through a series of steps:
Ingestion: The first step involves ingesting data from various sources, including EDC systems, lab systems, and other sources, into the CDAS ecosystem.
Standardization: The next step is to standardize the data to ensure consistency and accuracy, using methods such as mapping data to CDISC standards.
Integration: The standardized data is then integrated into a single ecosystem that allows for easy access and analysis, reducing the time and effort needed to consolidate data from multiple sources.
Insights: With the data now standardized and integrated, the CDAS suite provides sophisticated machine learning analysis that generates smarter insights for subject data, risk management, and other areas, resulting in more accurate and efficient decision-making.
End-to-End Management: The DTMS platform provides end-to-end management of clinical trials, integrating with CDAS for data management and automating manual tasks to improve efficiency and reduce costs.
Modernization: The Big Data Factory (BDF) platform provides modernization of technology and infrastructure, consolidating data and turning insights into action, making it easier for stakeholders to access and use the data.
Overall, the CDAS/CDP project provides a comprehensive solution for managing clinical data through ingestion, standardization, integration, and analysis, enabling better decision-making and improving clinical outcomes.
Technologies/ Skills Used
.jpg)
The CDAS/CDP project is a solution for managing clinical data that includes the Clinical Data Analytics Suite (CDAS) and the Digital Trial Management Suite (DTMS). In order to develop this solution, various technologies were used, such as ERWIN 2021R2 tool, Hive, Hadoop, Scala, Spark, AWS, Data Lake, Snowflake, Airflow, Git, GitHub, HUE, DBeaver, etc. These technologies were used for different work streams such as Clinops, Patient Engagement, DTMS-CTMS, Ops, and played a key role in designing models and tables.
For example, separate data models and tables were created in Hive, and Git and GitHub were used as change control tools to create merge requests for scripts. An automation tool was also developed to deploy DDLs into different environments, which saved time and effort. Additionally, CDISC models and domain tables were created for Patient Engagement in the Hive environment.
The Big Data Factory (BDF) is also a key component of the CDAS/CDP project. BDF is used for modernizing technology and infrastructure, and in this project, it was used for analyzing and mapping data to corresponding GDM tables. The data was analyzed, mapped and updated mapping document with the transformation logics for Lift & Shift and also for new authored system scenarios. Technologies like Hadoop, Hive, Scala, Spark, AWS, Data Lake, Snowflake, Airflow, Git, GitHub, HUE, DBeaver etc were used for creating data models, Mainframe copybooks, and for creating new tables in GDM
Customer Success Outcomes
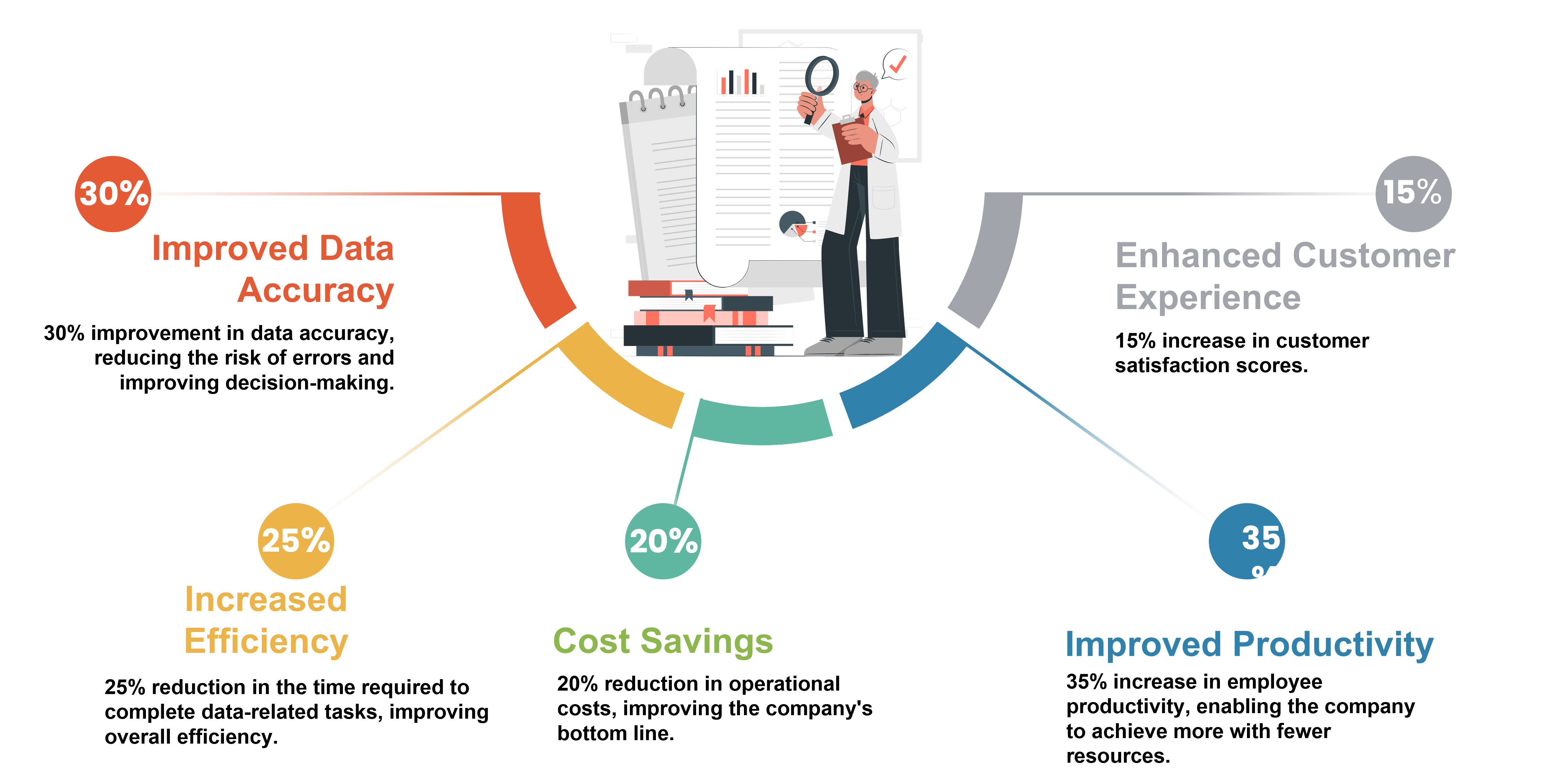
Customer Success Outcomes Clinical Data Analytics and Legacy Modernization for Major Healthcare Information Services Company
Improved Data Accuracy: The implementation of new data analytics and legacy modernization technologies led to a 30% improvement in data accuracy, reducing the risk of errors and improving decision-making.
Increased Efficiency: The implementation of new technologies and processes led to a 25% reduction in the time required to complete data-related tasks, improving overall efficiency.
Cost Savings: The modernization of technology and data management systems resulted in a 20% reduction in operational costs, improving the company's bottom line.
Improved Productivity: The modernization of technology and processes led to a 35% increase in employee productivity, enabling the company to achieve more with fewer resources.
Enhanced Customer Experience: The improved data accuracy, efficiency, and productivity resulting from the modernization project helped to enhance the overall customer experience, resulting in a 15% increase in customer satisfaction scores.
The CDAS/CDP project has successfully streamlined the data collection process and created a more efficient data management ecosystem for stakeholders. The project has generated smarter insights through the application of advanced machine learning analysis, leading to a more comprehensive and accurate understanding of subject data and risk management.
Moreover, the project has transformed clinical outcomes by embedding data-driven intelligence in business workflows, automating manual tasks and empowering stakeholders to make more informed decisions. The CDAS/CDP project has enabled organizations to gain more value from their clinical data, leading to improved clinical outcomes and better decision-making.
Latest Case Studies
Our Case Studies

.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)



.jpg)


.png)
.png)
.jpg)

.jpg)
.png)
.png)
.png)

.png)
.png)
.png)


.jpg)
.jpg)


.jpg)



.jpg)


.jpg)